October 2024
A busy month, with two days at New Scientist Live, one day at Quekex and two days at the National Honey Show. We had hundreds of visitors at New Scientist Live, with hardly a moment to sit down or eat sandwiches. We mostly used small digital microscopes with built-in screens. Gordon Brown attracted a lot of interest with the pseudoscorpions that he extracted from compost and showed on a monitor.

Quekett stand at New Scientist Live (with me on the right)
Quekex at Potters Bar was much quieter, so there was plenty of time to admire the exhbits and catch up with old friends.
The National Honey Show at Sandown Park is much less busy than New Scientist Live, but with surges of visitors between lectures and workshops. I took my Olympus SZ4045 stereomicroscope to show a honeybee and a wasp, and my Lenovo laptop to show PowerPoint presentations of photomicrographs by Quekett members.

PowerPoint presentation and stereomicroscope

Honeybee and German wasp
September 2024
There is no electricity available for the stalls at the Grove Park Carnival, so I used a powerpack with an LED ring-light on my Olympus SZ4045 stereomicroscope. The new wetland at Chinbrook Meadows is almost finished, and Thames21 officially opened it during the Carnival. There are four new ponds that have been colonised very quickly by all sorts of plants and animals, and I accompanied Nigel Ashby when he went collecting.

Nigel Ashby collecting from a duckweed-covered pond
As usual at this event, there were lots of enthusiastic youngsters with their parents, but we never really know what they manage to see.

Young visitors with small microscopes

Water slater, freshwater shrimp and mayfly nymph
For the Wimbledon Common Open Day on Sunday 8th, I planned to make use of the wall-mounted television in the Information Centre, like I used to do before other people started taking computers and monitors. I prepared two bags that I can carry on buses with my trinocular SZ4045 stereomicroscope and my Canon EOS 5D Mark II full-frame camera, with all the necessary adapters and a long HDMI cable, plus my EOS 600D and flash to take photographs for the meeting report. But the weather forecast kept getting worse, with heavy rain forecast all day, so I needed an umbrella and could only carry one bag with just the microscope, 600D and flash. Then the forecast turned out to be completely wrong, and the day was dry, warm and often sunny. Lots of families with small children came to see what Neil Henry and I collected from the new pond in the Wildlife Garden, but I wasn’t able to show the specimens on the television.

Neil Henry with visitors

Olympus SZ4045 stereomicroscope
I collected water and some filamentous algae (including Spirogyra), duckweed (Lemna minor) and water-milfoil (Myriophyllum) from the new pond, and found epiphytes on the duckweed roots, desmids (Closterium), ostracods, copepods, Cladocera, a small dragonfly nymph, and what I think were a microturbellarian and some small testate amoebae.
I went out to take photographs as soon as I arrived, because I was expecting the rain to start at any moment. It was quite cool, and on a flower above the pond I saw an insect that I did not recognise. It stayed still when I tried to photograph it, but I had to lean over the pond and so I could not get a good angle. Back home, Google Lens did not find anything similar, so I tried the image search in Microsoft Bing, and it suggested Bombus pascuorum, the common carder bee.

Common carder bee
August 2024
I went on the Quekett excursion to Warnham Local Nature Reserve on 3rd August. The forecast was for rain so I didn’t take a microscope because of the 25-minute walk from the station. On the day, there was hardly any rain, and there were lots of small organisms for which my stereomicroscope would have been useful. Graham Matthews was there with his trinocular Leitz Dialux, making it look easy to take stacked photomicrographs of living specimens.

Graham Matthews photographing Spirogyra
I have been trying to label all of the objectives, eyepieces and other accessories for my microscopes, partly so that I can keep track of things and partly for when the time comes to pass them on to someone else. I found a few items that I no longer need, and I hoped to sell them at Microscopium.

Items to sell at Microscopium
However, on the day I only managed to get as far as Finsbury Park, where I found that there were no trains to Letchworth Garden City because of damaged overhead wires, and there were no replacement buses. Now I will have to keep everything until the sales meeting in Reading next year.
July 2024
When I bought my Olympus BHMJ metallurgical microscope back in 2016, I hoped to be able to use it with a BH2-TR30 trinocular head. Unfortunately, this head is so heavy that the coarse focus drifts downwards, even with the tension set very high. The instruction manual even says “For photomicrography, it is preferable to clamp the tension adjustment ring tightly to prevent the microscope body from dropping during operation.”. This has not been a problem for me because I prefer using the BH2-MA Brightfield Vertical Illuminator that came with the BHMJ on my BHT stand. However, while planning what to take to the photomicrography workshop for Positive View, I wondered if using a lighter head would solve the problem. So I assembled my BH-BI30 binocular head, my BH-RLA vertical illuminator and my set of 5×, 10×, 20× and 40× Neo objectives.

BHMJ with BH-RLA vertical illuminator and Neo objectives
Sadly, even with a much lighter head and without a camera, this arrangement was also too heavy and the focus drifted. So now I will have to get out my No. 1 JIS screwdriver and remove the dovetail from the BH-NRE nosepiece so that I can screw it to my CH-2 stand. This stand has a more conventional arrangement where the focus only moves the stage and the condenser, and so the weight of the camera and head are not a problem.
For the workshop in the Angela Marmont Centre, I took my CH-2, Neo objectives, BH-RLA vertical illuminator and retroDIODE Obh10B LED conversion. I also took my Canon EOS 5D Mark II and my Lenovo laptop with EOS Utility.

Trinocular Olympus CH-2 with BH-RLA vertical illuminator and Neo objectives
For the meeting report, I wanted to include examples of the sorts of specimens that can be photographed using stereo and compound microscopes. I mostly used photomicrographs from other pages on the Quekett website, but I also took a couple of new subjects using a shadowless illuminator:

Geode with shadowless illuminator
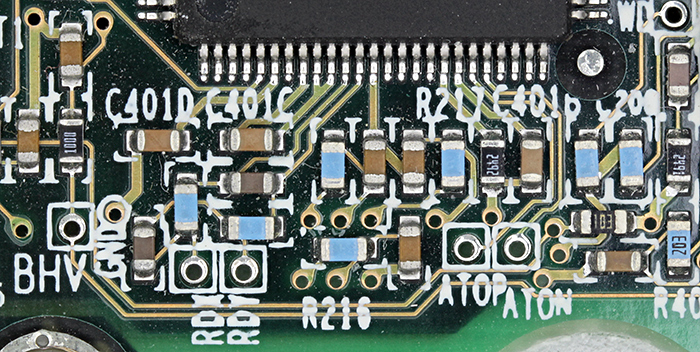
Printed circuit with shadowless illuminator
June 2024
I went to Wimbledon Common on Saturday 22nd June for the butterfly and dragonfly walk, part of their Weekend of Nature, and took several photos using my Canon EOS 600D camera and the standard 18-55 mm zoom lens.

Speckled wood (Pararge aegeria)

Rusty tussock moth (Orgyia antiqua) caterpillar on oak

Large red damselfly (Pyrrhosoma nymphula)

Newly-emerged (teneral) damselfly

Bumblebee

Honeybee (Apis mellifera)

Bee orchids (Ophrys apifera)
I went again the next day when the Quekett was participating as part of their outreach programme. Neil Henry and I set up our microscopes, cameras and computers in the open-fronted garage and showed an assortment of pond life, insects and plants from the Common to lots of visitors.
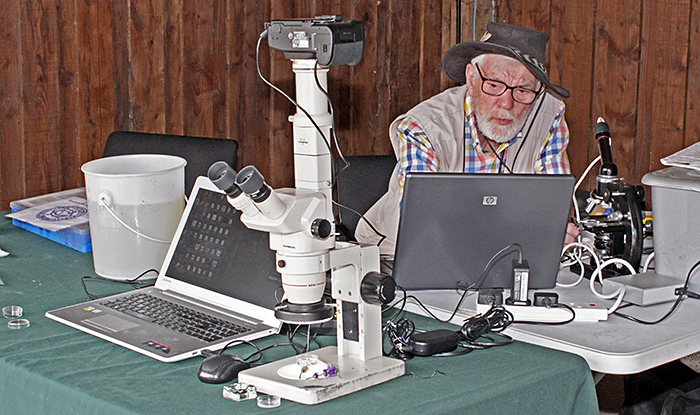
Microscopes, cameras and computers
With just two of us, I couldn’t go on the general nature walk; we really need more volunteers to help with the Quekett’s microscopy outreach programme. However, I had more time to take photos through my trinocular Olympus SZ4045 stereomicroscope, using my Canon EOS 5D Mark II controlled by EOS Utility on my laptop computer. Illumination was provided by my 144-LED ring-light.

Aphids on grass

Grass flowers

Green oak tortrix moth (Tortrix viridana)
The next weekend, I went on the Quekett excursion to Keston Ponds on Keston Common. I have not been there before, and it is a long journey into London by train and out again by train and bus, but it is a good location for pond-dipping. I went pond-dipping with Nigel Ashby and Jane Linstead, and we found lots of interesting specimens.

Keston Ponds

Jane Linstead with pipette

Nigel Ashby with plankton net
Back in the Village Hall, we watched David Linstead taking lots of photomicrographs, and making it look easy to get good results.

David Linstead with trinocular Zeiss Standard microscope
May 2024
I went to the workshop on Victorian slides in the Angela Marmont Centre on Tuesday 14th May. I don’t have any slides that are definitely Victorian, but I found three that might be Victorian, although none of them are marked with the mounter or a date. One was a roughly-cut glass slide (79 × 27.5 × 2 mm) of Pulex irritans, one was a wooden slide of dry mounted Lagena forams from Sligo, and the third was a small (58.5 × 18 mm) paper-covered glass slide of a cat flea.

Victorian slides
I had another go at photographing scales on the wing of a small heath butterfly, this time using a normal Olympus A20 objective with diffuse incident light. The A 20 has a working distance of 1.63 mm, slightly more than the DPlan 20 (0.83 mm) and the SPlan 20 (1.50 mm). I used two Ikea Jansjo lights close to a white paper cylinder, but the light was so dim thought the eyepieces and on the computer screen that it was extremely difficult to focus.

Scales of small heath butterfly
[Olympus A 20 objective, NFK 2.5 photo eyepiece, Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 93 images, 2 µm steps, combined in Zerene Stacker PMax.]

Diffused incident light
The results are still nowhere near as good as I had hoped. Now I understand why the people in the forums on photomacrography.net use very expensive high-magnification objectives with long working distances that enable them to get good resolution and have enough space for high-intensity lights.
I experimented with with various sizes of cylindrical and conical diffusers made of white paper, but none of them provided anywhere near enough light.
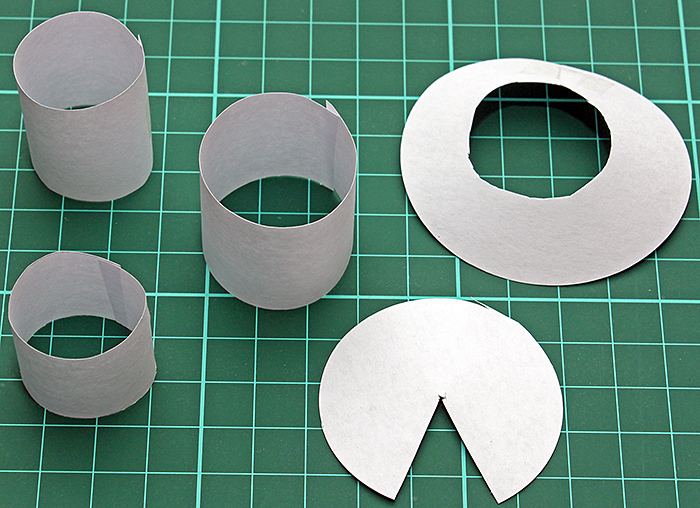
Cylindrical and conical diffusers
April 2024
Back in the days before digital cameras tethered to computers, nearly all 35 mm camera manufacturers and several accessory manufacturers sold simple adapters that clamped onto an eyepiece tube with a 25 mm outside diameter. They separated into two parts, so that you could insert a microscope eyepiece. There was no image stacking in those days, so I think they must have expected an ordinary eyepiece to produce an image on the film that was reasonably sharp into the corners. I recently tested some of my eyepieces with one of these old-fashioned microscope adapters that has a T-mount to allow it to be used with most cameras. I found that the Zeiss Kpl 8×/18 I acquired at Reading last month gave very good results on my old Canon EOS 40D (APS-C sensor), with the image sharp right into the corners. I also tested an afocal arrangement with a Leitz Periplan 10/18 on a Zuiko 50 mm f/1.8 standard lens, which gave a slightly wider field of view than the Zeiss, but the extreme corners are not quite sharp. The objective was an Olympus SPlan 4.
The eyepieces that I tested with the T-mount adapter were:
- Olympus NK 5× L (widest field of view, poor away from the centre, vignetting caused by field iris)
- Olympus NFK 2.5 LD 125 photo eyepiece (wider field of view than Zeiss, but corners not sharp)
- Zeiss Kpl 8×/18 46 39 20 - 9902 (reasonable field of view, corners sharp)
- unbranded W.F. 10× (smaller field of view than Zeiss, corners not quite sharp)
Field of view of eyepieces tested with T-mount adapter
[Top left: NK 5, Top right: NFK 2.5, Bottom left: Kpl 8, Bottom right: W.F. 10]
Leitz Periplan 10/18 on a Zuiko 50 mm f/1.8 standard lens
All of the eyepieces give good images when used normally, but they were not designed for use with a T-mount adapter, and the only way to find out if they work well is to test them. The T-mount adapter places the sensor 102 mm from the end of the eyepiece tube. The NFK is designed to produce a real image at a distance of 150 mm, and the others are only designed to produce a virtual image.
I would have liked to test my Olympus CWHK 10×/18 L, Olympus WHK 10×/20 L and Leitz Periplan 10/18, but they are too wide to fit in the adapter. The adapter cannot be used with my binocular Olympus BH-2 and CH-2 heads, because their eyepiece tubes are too wide.
I took the Leitz afocal arrangement to the workshop on stains and staining in the Natural History Museum, led by Terry Hope, prepared to take some photomicrographs of the slides made by the participants. However, when I got to the Angela Marmont Centre I realised that I would not be able to take any photomicrographs, because their microscopes are all binocular and the eyepieces are held in place with locking screws.
Back in 2015, Kai and I went to visit Susanna Ramsay (Nature Collection) in Kingston, to help her get the best photographs through the trinocular stereomicroscope that David Linstead and Ray Sloss had helped her buy. Susanna has been in touch again, asking for advice on a good trinocular compound microscope that would be suitable for photographing the scales on butterfly wings. This is something that I have not tried before, and I knew that it would need reflected light, so I had a go with my BH reflected light adapter and a Neo 20× objective. The wing that I tried had hairs as well as scales, and needed a stack of 103 images.

Scales of small heath butterfly
[Olympus Neo 20 objective, FK 2.5 photo eyepiece, Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 103 images, 2 µm steps, combined in Zerene Stacker PMax.]
I couldn’t use my Neo 40 objective because its working distance is too short. Susanna won’t want to use a reflected-light attachment, and she wants a higher magnification. I think a long-working distance 40× objective with a correction collar (designed for an inverted microscope) might be suitable, but I have to work out what lighting to use. A microscope that uses infinity-corrected objectives with a long-working distance metallurgical objective might also be suitable, but would be too expensive.
March 2024
The Reading Convention is now being run by the Quekett and has been re-named the Quekett Spring Sale. It is an awkward journey to Sonning Common, 3¾ hours by public transport, but it is worth it to meet up with old friends, sell a few items, and use the proceeds to buy some small items. I sold some Biosil slides of crystals and trematodes, and the Meopta AZ-2 portable microscope that I bought several years ago but have never used. I picked up a Zeiss Kpl 8×/18 eyepiece and an Olympus NK 5× L eyepiece, and when I have time I will try them out for photomicrography with a simple camera adapter. I also bought something I thought I would never find, an adjustable Olympus sector diaphragm for oblique dark-ground reflected illumination with my BH-era Neo objectives.
Back to normal for the workshop on hair slides in the Natural History Museum, led by John Gregory. I was too busy taking photographs and notes to make any slides, but I was able to take some of John’s demonstration slides home to photograph.
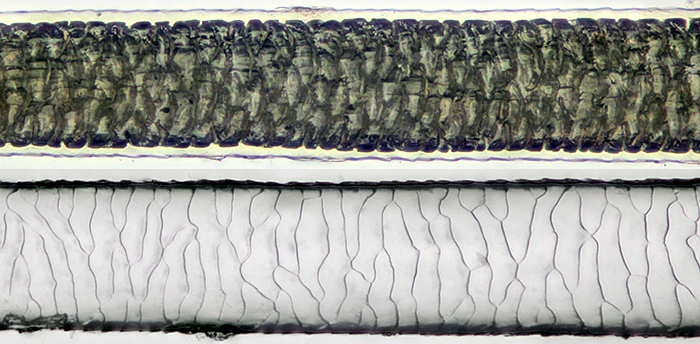
Guinea pig hair mounted in LOCA (top) and impression in LOCA
[Olympus SPlan 20 objective, Nikon Achr 0.85 condenser, NFK 2.5 photo eyepiece. Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 18 images, 2 µm steps, combined in Zerene Stacker PMax. Hair diameter 0.07 mm]

Human hair mounted in LOCA
[Olympus SPlan 20 objective, Nikon Achr 0.85 condenser, NFK 2.5 photo eyepiece. Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 12 images, 2 µm steps, combined in Zerene Stacker PMax. Hair diameter 0.08 mm]
February 2024
I am usually too busy taking photographs and notes to actually participate in Quekett workshops, but I managed to make some slides at the workshop on practical slide-mounting in the Natural History Museum, led by Chris Thomas.

Dry-mounted slides
Chris provided a saturated solution of vitamin C and demonstrated applying a small drop to a slide. The drop can be left to dry, or it can be spread out thinly. Crystal growth can be encouraged by adding small crystals as seeds, or by scratching the deposit while it is still damp. I didn’t spread my drop, and other people got better results with thin layers.
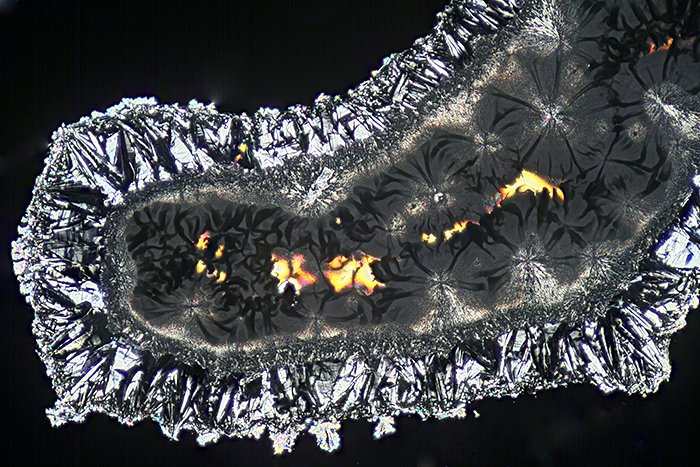
Vitamin C crystals
Chris showed us how to paint a circle of diluted PVA glue on a slide using a ringing turntable. Then sprinkle sand onto the glue, turn the slide over and tap off the excess sand. The sand can be protected by adding a 3″×1″ piece of 1 mm card with a central hole.

Orkney sand (protected with card cover)

Orkney sand
Chris showed us a simple method for finding the centre of a slide: draw round the slide, then draw diagonals, and the centre is where the diagonals cross.
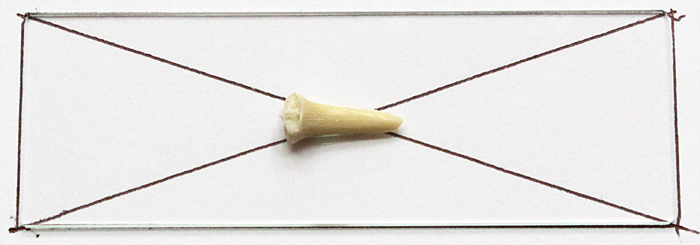
Guide for positioning shark tooth
To fasten the tooth to the slide, we used a tiny drop of Vida Rosa UV Resin, cured by holding the slide over a UV torch for 60 seconds.

Shark tooth
Chris demonstrated a method for mounting a thin specimen between two slides that are held together with 6 mm copper tape. The idea came from Michael Horwood, and Gordon Brown recommended the spring clips that hold the slides while the tape is being applied.

Blackbird feather between two slides
I also went to the Home Counties Meeting in Cobham, a simple journey by bus for me. I took a selection of home-made gadgets for holding specimens under a stereomicroscope or a camera with a macro lens, but there were so many other interesting exhibits that I kept mine for another occasion.
January 2024
Barry Wendon, Paul Smith and I took microscopes and specimens to the “Life Under a Microscope” session of the Wimbledon Common Nature Club. It is run by Auriel Glanville and her assistants (Luci Teuma and Alexander and Oliver Mallett) and welcomes children from 6–14 years old to come and discover the world of nature on the Common. They meet for 2 hours each month in the Information Centre, the same venue as used by Quekett members on excursions, the Weekend of Nature and the Open Day. I took my Olympus SZ4045 stereomicroscope with reflected and transmitted light, and a small and simple stereomicroscope.

Stereo microscopes
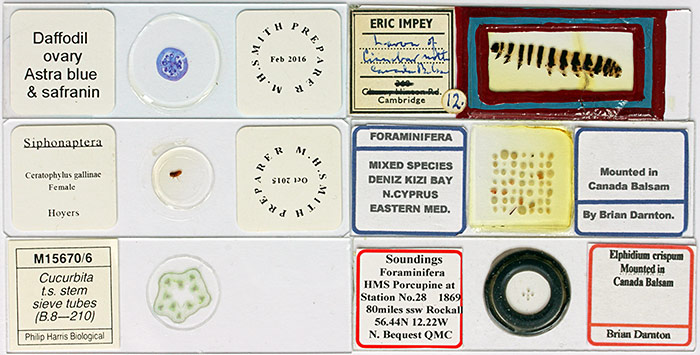
Microscope slides

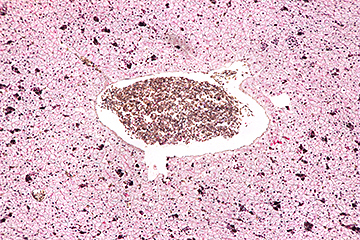
Arrangement of foraminifera
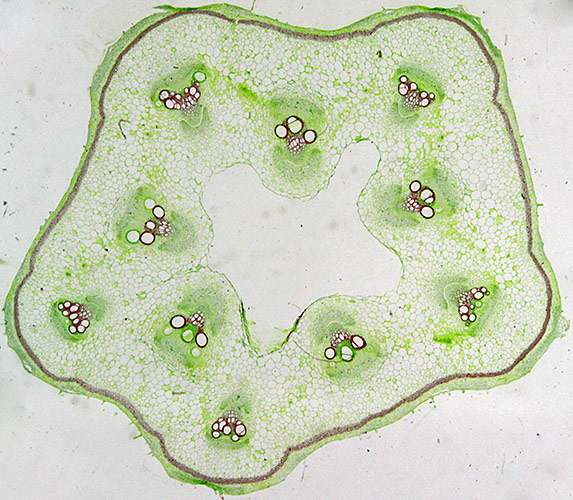
Sieve tubes in Cucurbita sp.
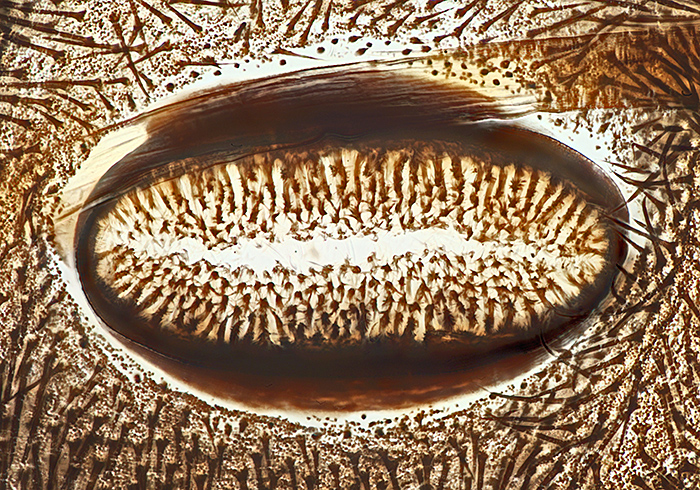
Spiracle of cinnabar moth caterpillar (from slide of whole skin)
Commenting on this blog
If you would like to comment on anything in this blog, please send me a message.