April 2024
I still do not have a really good way of taking photomicrographs at Quekett meetings, so I recently tested some of my eyepieces with an old-fashioned T-mount microscope adapter that clamps onto an eyepiece tube with a 25 mm outside diameter. I found that the Zeiss Kpl 8×/18 I acquired at Reading last month gave very good results on my old Canon EOS 40D (APS-C sensor), with the image sharp right into the corners. An afocal arrangement with a Leitz Periplan 10/18 on a Zuiko 50 mm f/1.8 standard lens has a slightly wider field of view, but the extreme corners are not quite sharp. The objective was an Olympus SPlan 4.
The eyepieces that I tested with the T-mount adapter were:
- Olympus NK 5× L (widest field of view, poor away from the centre, vignetting caused by field iris)
- Olympus NFK 2.5 LD 125 photo eyepiece (wider field of view than Zeiss, but corners not sharp)
- Zeiss Kpl 8×/18 46 39 20 - 9902 (reasonable field of view, corners sharp)
- unbranded W.F. 10× (smaller field of view than Zeiss, corners not quite sharp)
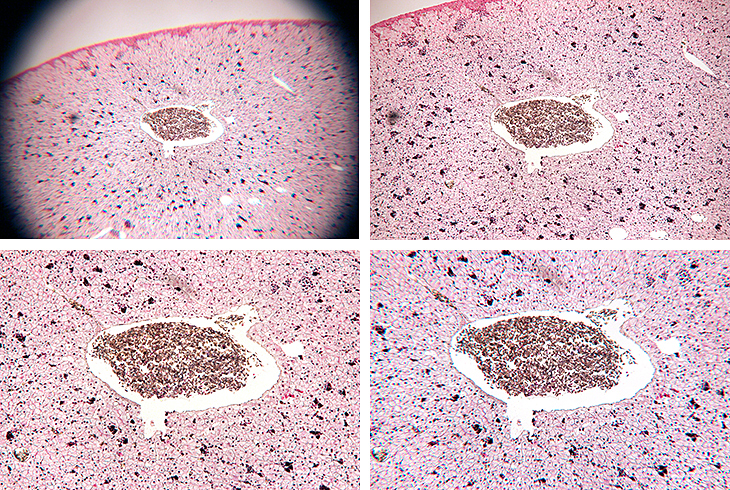
Eyepieces tested with T-mount adapter
[Top left: NK 5, Top right: NFK 2.5, Bottom left: Kpl 8, Bottom right: W.F. 10]
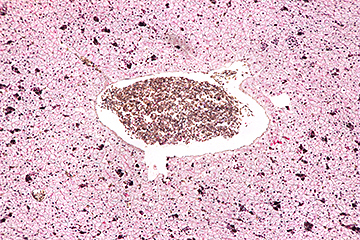
Leitz Periplan 10/18 on a Zuiko 50 mm f/1.8 standard lens
All of the eyepieces give good images when used normally, but they were not designed for use with a T-mount adapter, and the only way to find out if they work well is to test them. The T-mount adapter places the sensor 102 mm from the end of the eyepiece tube. The NFK is designed to produce a real image at a distance of 150 mm, and the others are only designed to produce a virtual image.
I would have liked to test my Olympus CWHK 10×/18 L, Olympus WHK 10×/20 L and Leitz Periplan 10/18, but they are too wide to fit in the adapter. The adapter cannot be used with my binocular Olympus BH-2 and CH-2 heads, because their eyepiece tubes are too wide.
I took the Leitz afocal arrangement to the workshop on stains and staining in the Natural History Museum, led by Terry Hope, prepared to take some photomicrographs of the slides made by the participants. However, when I got to the Angela Marmont Centre I realised that I would not be able to take any photomicrographs, because their microscopes are all binocular and the eyepieces are held in place with locking screws.
Back in 2015, Kai and I went to visit Susanna Ramsay (Nature Collection) in Kingston, to help her get the best photographs through the trinocular stereomicroscope that David Linstead and Ray Sloss had helped her buy. Susanna has been in touch again, asking for advice on a good trinocular compound microscope that would be suitable for photographing the scales on butterfly wings. This is something that I have not tried before, and I knew that it would need reflected light, so I had a go with my BH reflected light adapter and a Neo 20× objective. The wing that I tried had hairs as well as scales, and needed a stack of 103 images.

Scales of small heath butterfly
[Olympus Neo 20 objective, FK 2.5 photo eyepiece, Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 103 images, 2 µm steps, combined in Zerene Stacker PMax.]
I couldn’t use my Neo 40 objective because its working distance is too short. Susanna won’t want to use a reflected-light attachment, and she wants a higher magnification. I think a long-working distance 40× objective with a correction collar (designed for an inverted microscope) might be suitable, but I have to work out what lighting to use. A microscope that uses infinity-corrected objectives with a long-working distance metallurgical objective might also be suitable, but would be too expensive.
March 2024
The Reading Convention is now being run by the Quekett and has been re-named the Quekett Spring Sale. It is an awkward journey to Sonning Common, 3¾ hours by public transport, but it is worth it to meet up with old friends, sell a few items, and use the proceeds to buy some small items. I sold some Biosil slides of crystals and trematodes, and the Meopta AZ-2 portable microscope that I bought several years ago but have never used. I picked up a Zeiss Kpl 8×/18 eyepiece and an Olympus NK 5× L eyepiece, and when I have time I will try them out for photomicrography with a simple camera adapter. I also bought something I thought I would never find, an adjustable Olympus sector diaphragm for oblique dark-ground reflected illumination with my BH-era Neo objectives.
Back to normal for the workshop on hair slides in the Natural History Museum, led by John Gregory. I was too busy taking photographs and notes to make any slides, but I was able to take some of John’s demonstration slides home to photograph.
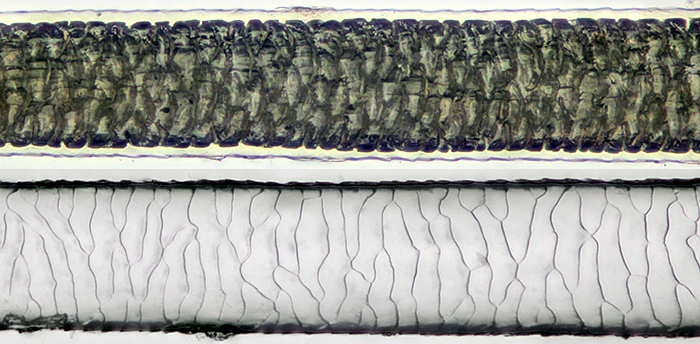
Guinea pig hair mounted in LOCA (top) and impression in Vida Rosa
[Olympus SPlan 20 objective, Nikon Achr 0.85 condenser, NFK 2.5 photo eyepiece. Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 18 images, 2 µm steps, combined in Zerene Stacker PMax. Hair diameter 0.07 mm]

Human hair mounted in LOCA
[Olympus SPlan 20 objective, Nikon Achr 0.85 condenser, NFK 2.5 photo eyepiece. Canon EOS 5D Mark II controlled by EOS Utility, ISO 200. Stack of 12 images, 2 µm steps, combined in Zerene Stacker PMax. Hair diameter 0.08 mm]
February 2024
I am usually too busy taking photographs and notes to actually participate in Quekett workshops, but I managed to make some slides at the workshop on practical slide-mounting in the Natural History Museum, led by Chris Thomas.

Dry-mounted slides
Chris provided a saturated solution of vitamin C and demonstrated applying a small drop to a slide. The drop can be left to dry, or it can be spread out thinly. Crystal growth can be encouraged by adding small crystals as seeds, or by scratching the deposit while it is still damp. I didn’t spread my drop, and other people got better results with thin layers.
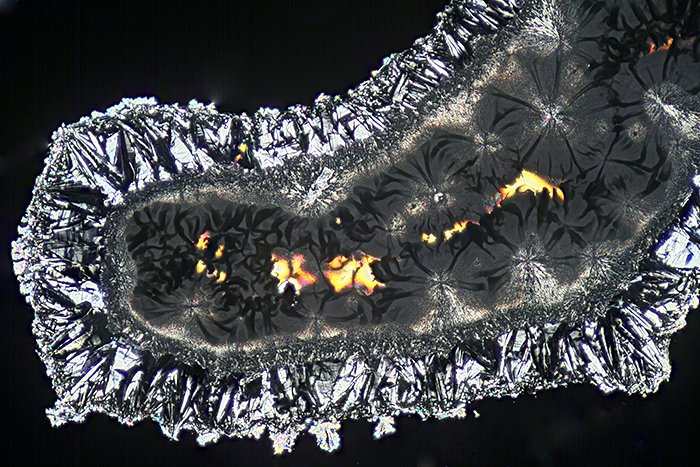
Vitamin C crystals
Chris showed us how to paint a circle of diluted PVA glue on a slide using a ringing turntable. Then sprinkle sand onto the glue, turn the slide over and tap off the excess sand. The sand can be protected by adding a 3″×1″ piece of 1 mm card with a central hole.

Orkney sand (protected with card cover)

Orkney sand
Chris showed us a simple method for finding the centre of a slide: draw round the slide, then draw diagonals, and the centre is where the diagonals cross.
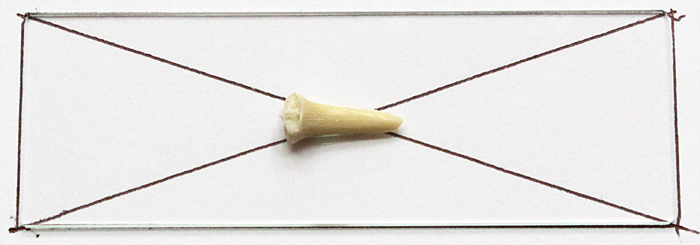
Guide for positioning shark tooth
To fasten the tooth to the slide, we used a tiny drop of Vida Rosa UV Resin, cured by holding the slide over a UV torch for 60 seconds.

Shark tooth
Chris demonstrated a method for mounting a thin specimen between two slides that are held together with 6 mm copper tape. The idea came from Michael Horwood, and Gordon Brown recommended the spring clips that hold the slides while the tape is being applied.

Blackbird feather between two slides
January 2024
Barry Wendon, Paul Smith and I took microscopes and specimens to the “Life Under a Microscope” session of the Wimbledon Common Nature Club. It is run by Auriel Glanville and her assistants (Luci Teuma and Alexander and Oliver Mallett) and welcomes children from 6–14 years old to come and discover the world of nature on the Common. They meet for 2 hours each month in the Information Centre, the same venue as used by Quekett members on excursions, the Weekend of Nature and the Open Day. I took my Olympus SZ4045 stereomicroscope with reflected and transmitted light, and a small and simple stereomicroscope.

Stereo microscopes
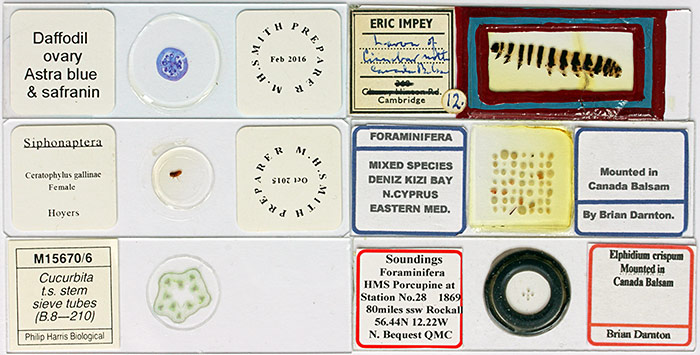
Microscope slides

Arrangement of foraminifera
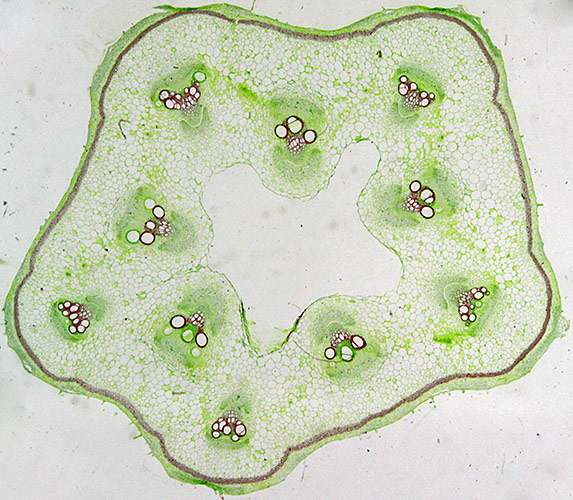
Sieve tubes in Cucurbita sp.
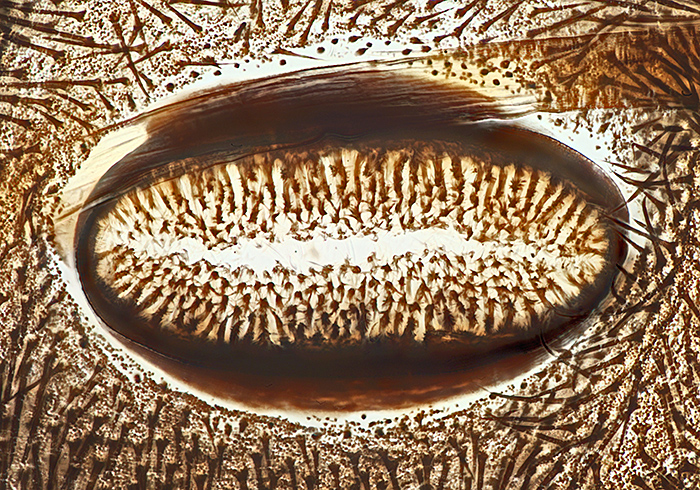
Spiracle of cinnabar moth caterpillar (from slide of whole skin)
Commenting on this blog
If you would like to comment on anything in this blog, please send me a message.