December 2017
I only used a microscope once this month, when the strap on my wife’s wristwatch came off. My Olympus SZ4045 stereomicroscope was the ideal tool for this job, with the 0.62× supplementary objective to give a wide enough field of view.
I have made a little progress on my Powerpoint on image processing.
November 2017
I am going to be very busy with my other life (pesticide nomenclature) for the next year or so, and I am struggling to find the time for Quekett matters. Graham Matthews and I ran a workshop on image processing and I didn’t have time to finish my Powerpoint presentation so I showed some things live. I must find the time to finish the presentation.
The workshop for Arkwright Scholars was another busy day with a dozen enthusiastic and very bright sixth-formers, and I still have to write a report on it.
October 2017
I submitted 3 photos for a Barnard Award, and was surprised that the one of fern sori received a certificate at the Annual Exhibition of Microscopy. It was taken just before the deadline and I didn’t take the time to diffuse the lighting.

Sori on underside of a fern frond
Olympus Zuiko Auto-Macro Lens 38mm f/2.8, used at f/4, magnification 5× on sensor of Canon EOS 5D Mark II, illumination from 2 desk lamps
I also posted it on the Ferns, lichens and mosses group on Facebook, and received some nice comments.
September 2017
Brown slime on rocks is one of the classic sources of live diatoms, and until recently a water feature in a local park had some interesting specimens. Now the Council has cut off the water supply and filled in the pool, so I have been looking for another source, and a wall underneath a railway bridge looked interesting.

Wall under a railway bridge

Brown slime and green algae
The green bits look like some sort of alga, growing on top of the brown slime.

Green algae
Olympus Neo 10× objective, FK 2.5× photo eyepiece, 61 images (0.008 mm steps) combined in Zerene Stacker
The brown slime yielded lots of diatoms, some gliding along and some stationary.

Diatoms from brown slime
Olympus SPlan 20× objective, NFK 2.5× photo eyepiece, 24 images (0.002 mm steps) combined in Zerene Stacker
Tony Pattinson identified this filamentous diatom for me, Melosira sp.

Melosira sp. from brown slime
Olympus SPlan 40× objective, NFK 2.5× photo eyepiece, 35 images (0.001 mm steps) combined in Zerene Stacker
I was surprised to see several spherical organisms about 0.1 mm diameter and I didn’t know what they were; Wim van Egmond suggested Botrydiopsis sp. or Bracteacoccus sp.
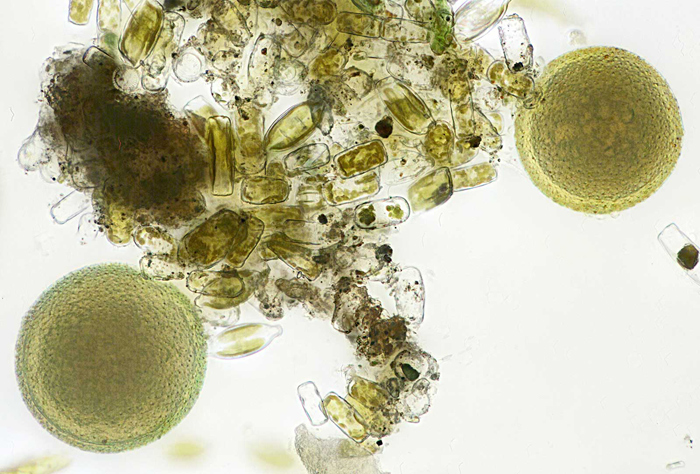
Spherical algae from brown slime
Olympus SPlan 40× objective, NFK 2.5× photo eyepiece, 30 images (0.001 mm steps) combined in Zerene Stacker
August 2017
I have often seen tar spot (caused by the fungus Rhytisma acerinum Schwein) on leaves of sycamore trees, but it looked black and featureless so I haven’t bothered to put it under a microscope. During the excursion to Warnham Local Nature Reserve I finally had a look at it, and it isn’t featureless after all.

Sycamore tar spot (the dark patch is about 10 mm across)
Canon EOS 600D with 60 mm EF/S macro lens at f/4.5, shadowless illumination from an inverted LED ring-light
I have a few dozen botanical slides, so I sorted out some interesting ones and photographed them for the “Botanical slides” gossip meeting. Starch grains under polarised light is one of the classic “wow” slides, but I haven’t photographed it before.
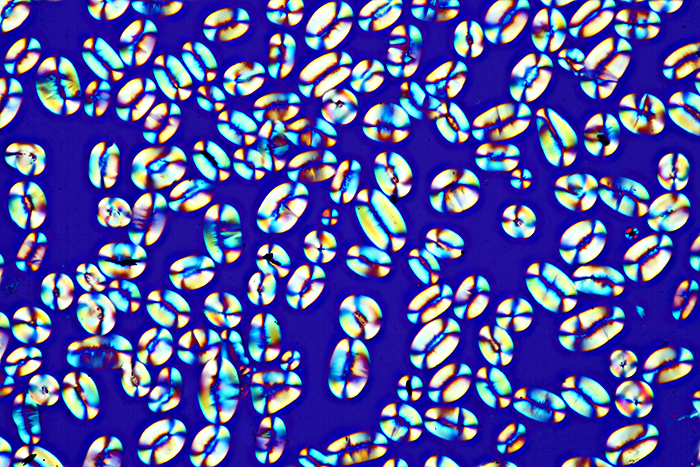
Starch grains from Calabar bean (Physostigma venenosum Balf.)
Olympus SPlan 20× objective, NFK 2.5× photo eyepiece, crossed polarisers with makeshift retarder, 14 images (0.002 mm steps) combined in Zerene Stacker
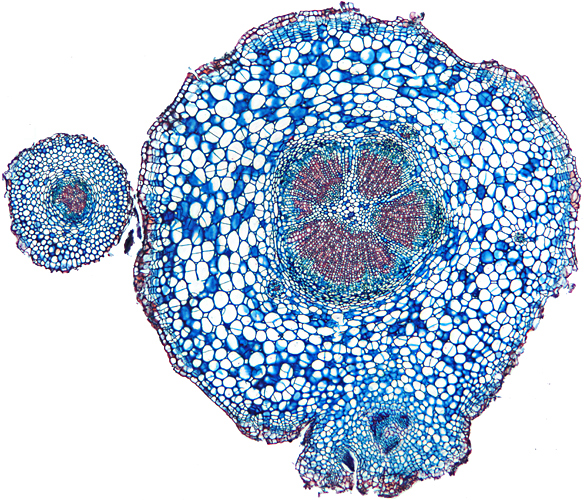
Transverse section of root of daisy (Bellis perennis L.), slide by John Wells (Biosil)
Olympus SPlan 4× objective, NFK 2.5× photo eyepiece, 10 images (0.03 mm steps) combined in Zerene Stacker
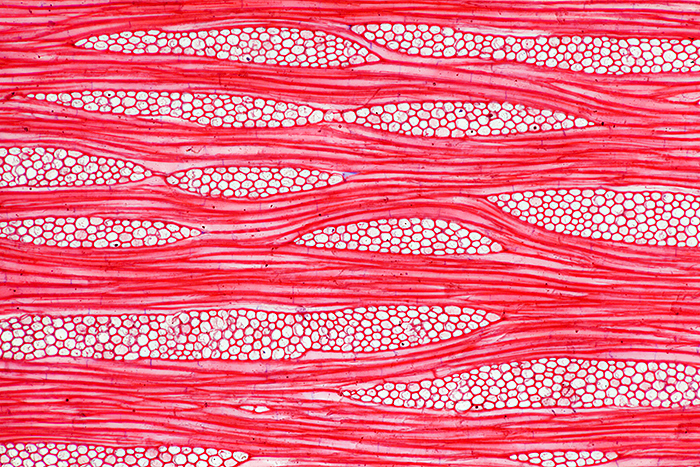
Tangential section of wood from teak (Tectona grandis L.f.), stained with safranin, slide by Ernie Ives
Olympus SPlanApo 10× objective, NFK 2.5× photo eyepiece, 23 images (0.002 mm steps) combined in Zerene Stacker
July 2017
As usual, the excursion to the Basingstoke Canal (based in Brookwood Memorial Hall) provided a really interesting day, with fascinating specimens, several microscopes to compare and admire, and lots of gossip. The excursions are relaxed events, and a good way to get to know other members.
Dennis Fullwood arranged a visit for a small group of Quekett members to the team in the Natural History Museum that is digitising their slide collection; they have millions of them. It was interesting to see that despite the availability of hugely expensive equipment, they prefer a workflow that images one slide at a time with Canon EOS digital SLRs, Tamron macro lenses and circular fluorescent lights.
For the gossip meeting on “My latest microscopical acquisition” I demonstrated a technique that I used for the first time this year, shadowless illumination from an upward-pointing LED ring-light covered by an inverted kitchen bowl with its base removed.

Bluebottle (Calliphora vomitoria (L.)) using shadowless illumination (body length 10.5 mm)
Canon EOS 5D Mark II, reversed Apo-Rodagon-N f=80mm enlarging lens at f/5.6, stack of 41 images at 200 µm intervals combined in Zerene Stacker
At the Northampton meeting last month, Steve Gill asked if it would be possible to load the archival series of Little Imp CD-ROMs onto the Quekett website, something that Brian Bracegirdle had requested. Fortunately the website hosting package that we use allows us to load an unlimited amount of data, so it sounded like a simple job. Unfortunately, modern web browsers don’t support all of the techniques that Steve used back in 2005, so he has had to update a lot of the HTML and JavaScript, and everything had to be tested. We got everything working, and 14 of Brian’s compilations of hard-to-find information on Beck, Watson and Zeiss microscopes and Enock and Clarke & Page slides are now available in the Little Imp Archival Series.
June 2017
Another month with hardly any time to actually look through a microscope. The Club is in the process of moving management of subscriptions from one man to an organisation, the Royal Society of Biology. They will need to be able to send out e-mails from a quekett.org address, and to enable this I had to register the Club with TechSoup and then with Google for Nonprofits and transfer all of the e-mail addresses to the Google system. This turned out to be far more time-consuming and complicated than I expected, and I had to learn how to set up a complicated Sender Policy Framework (SPF) record to help prevent e-mails being flagged as spam.
I attended and wrote reports for 3 events, the “Slides for polarised light” gossip, the Wimbledon Common Bioblitz (an outreach event), and the Annual Exhibition of the Microscopy Section of the Northamptonshire Natural History Society. It was my first visit to Northampton, and for some reason the first time it has been reported since 2002.
I spent one evening taking some photomicrographs for the gossip, with examples of bright-field, crossed polarisers and crossed polarisers plus a retarder.
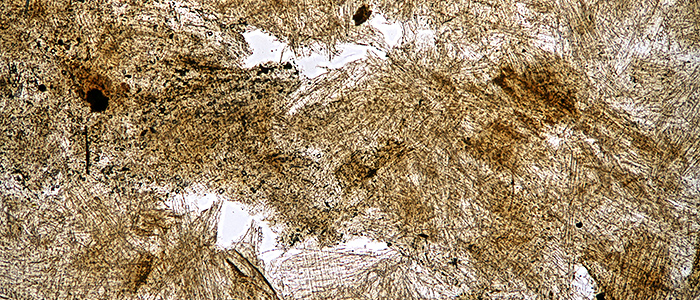
Wasp-nest paper (bright-field, Olympus SPlan 4× objective)
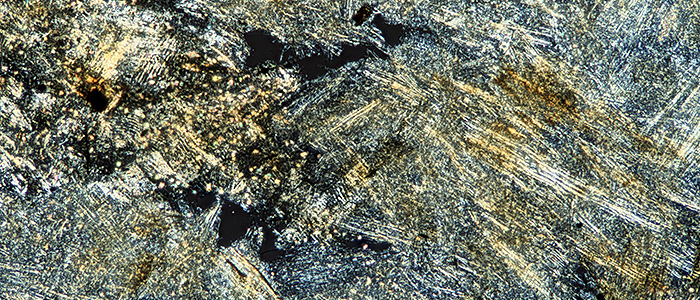
Wasp-nest paper (crossed polarisers, Olympus SPlan 4× objective)
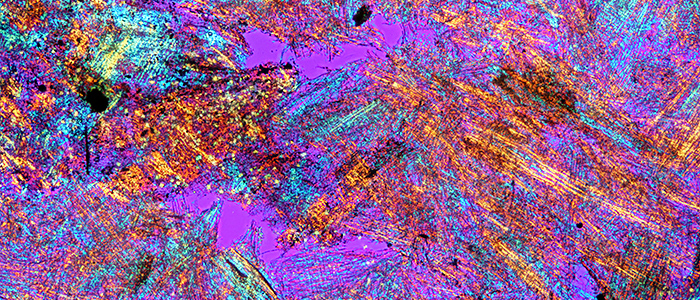
Wasp-nest paper (crossed polarisers + retarder, Olympus SPlan 4× objective)
The BioBlitz at Wimbledon Common is always fun, and during the guided walks I photographed some interesting specimens. If you have ever tried to photograph grasshoppers you will know that they usually hop away or shuffle round to the back of a stem, but I managed to get this shot:

Grasshopper on heather
Another subject I find difficult is bees, because they never stay still and it is difficult to get enough depth of field, but autofocus helps and I got this shot:

Honeybee on bird’s-foot trefoil
When I was at school, ponds used to have lots of tadpoles every year, but now they are much harder to find. I can’t remember the last time I saw a newt tadpole, but we caught 2 of them. They were not easy to see, being a similar colour to the debris, but I managed to get one reasonable photo:

Newt tadpole
May 2017
This has been a busy month, attending and writing reports for 3 all-day events, the PMS AGM at Husbands Bosworth (the first time I have been there), the excursion to Wimbledon Common, and a new event, a combined excursion and gossip at Eastbrookend Country Park.
I don’t seem to have had much time to use my own microscopes at home, but I got out my macro equipment to photograph some of the plant specimens that I showed under my Olympus SZ4045 stereomicroscope at Eastbrookend.

Burr of herb bennet (Geum urbanum L.)
Canon EOS 600D with 60 mm EF/S macro lens at f/4.5, stack of 21 images in Zerene Stacker (PMax), lit by 2 desk lamps with a Kleenex diffuser, burr is 22 mm across

Spines on stem of cleavers (Galium aparine L.)
Canon EOS 600D with 60 mm EF/S macro lens at f/4.5 plus 8-dioptre close-up lens, stack of 14 images in Zerene Stacker (PMax), lit by 2 desk lamps (no diffusion), stem is 1.4 mm across

Female catkin of crack willow (Salix fragilis L.)
Canon EOS 600D with 60 mm EF/S macro lens at f/4.5, stack of 38 images in Zerene Stacker (PMax), lit by 2 desk lamps with a Kleenex diffuser, catkin is about 10 mm across, green stem is about 0.7 mm across
I finally made the time to scan QMC Monograph No. 3 (Illuminants and Illumination for Microscopical Work by F. E. J. Ockenden) that Mike Samworth gave me at the Reading Convention, and the resulting PDF is now available on the Downloads page.
April 2017
I recently had to say goodbye to an old friend, the Olympus BHB that I bought several years ago with the intention of taking it to Quekett meetings and excursions. It had a trinocular head, some nice Plan objectives, the uncommon BH-SH rotatable stage, and all the bits for attaching a camera, but it turned out to be too heavy for me to carry on public transport. I now have an Olympus CHS that I take to meetings, and so I had to comply with my wife’s one-in-one-out policy and I sold the BHB to a couple who contacted the Quekett for advice on a microscope to use with their Canon EOS digital SLR.

Trinocular Olympus BH (BHB) outfit
Many years ago, I was given a small black weevil and asked to take a photograph showing its blue iridescence. This was in the days of film, and I tried my Olympus T8 Ring Flash 2 and I tried a pair of Bowens 750 Monolites firing into 40″ white umbrellas, but I never got a good result. Recently, Dennis Fullwood asked if I could help put on a microscopy display for the Amateur Entomologists’ Society Members’ Day at the Natural History Museum, and I thought I would have another go at photographing the weevil. I remembered seeing a description of a shadowless illuminator using an LED ring-light shining upwards into a white hemisphere. My first attempt was hopeless, spoiled by lots of flare, and then I remembered that there was supposed to be a barrier to keep direct light from the ring out of the lens, and a cylinder of white paper fixed the problem.

Shadowless illuminator, showing (top left) specimen pinned into a Plastazote disc, (top right) ring of white paper, (bottom left) inverted bowl with base removed, and (bottom right) the complete illuminator with a baffle of white foam-board.
I also took a photo using a desk lamp, to show the difference that lighting can make.

Small weevil using a desk lamp (left) and a shadowless illuminator (right)
For the display at the NHM I used my Olympus SZ4045 stereomicroscope, but to take the photographs I used my Canon EOS 5D Mark II with Olympus OM Telescopic Auto Tube 65–116, Zuiko Auto-1:1 Macro 80mm f/4 and Close-up Lens 80mm Macro f=170 mm. The magnification on the sensor was 2×, the lens was stopped down to f/5.6, and I took 57 images at 50 µm intervals and combined them in Zerene Stacker. Then I used Photoshop Elements 11 to crop, adjust levels and sharpen the image. I still haven’t managed to light the complete surface of the elytra, but the photo and the view through the microscope did impress lots of entomologists.
March 2017
The Olympus BH-RLA vertical illuminator for brightfield/darkfield and BH-LHM lamp house came with an Olympus TE-II transformer that is so heavy that it has prevented me from taking it to any Quekett meetings. Now I have a Obh10B LED illuminator from retroDIODE that is much lighter and I used it for a demonstration at the South Thames Discussion Group meeting at Wallington on 4th March.
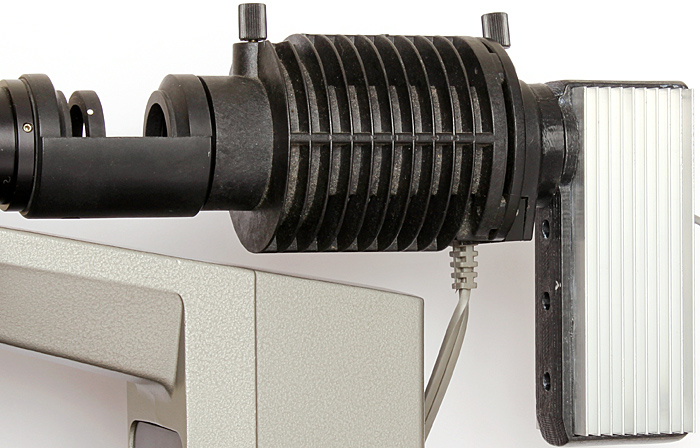
LED conversion of Olympus 15 watt BH-LHM lamp house
In reflected darkfield illumination, light to illuminate the specimen is piped down a cylinder surrounding the Neo objectives, so they are too wide for an RMS nosepiece and need a special one with 25 mm threads. The CH-2 that I wanted to use at the meeting does not have an interchangeable nosepiece, but it is easy to unscrew it and replace it with the 25 mm version.
The darkfield mode works well with biological specimens including feathers, lichen and pollen on stamens, but the shallow depth of field (around 0.05 mm for the 5× NA 0.1 objective) means that I have to keep refocusing to see all of a specimen. I took series of photographs of the specimens, and combined them using Zerene Stacker, to produce photographs with good depth of field.

Lichen-encrusted twig under the microscope
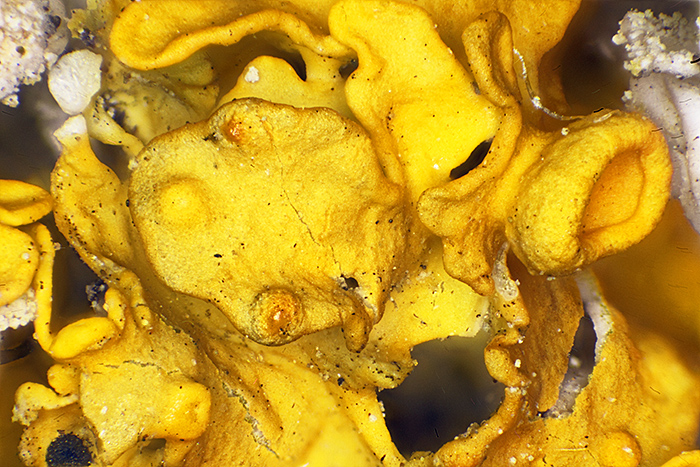
Stacked image of yellow lichen (Olympus Neo 5× objective, FK 2.5× photo eyepiece, stack of 44 images, 1.75 mm depth of field)

Stacked image of crocus pollen on stamen (Olympus Neo 5× objective, FK 2.5× photo eyepiece, stack of 42 images, 1.7 mm depth of field)
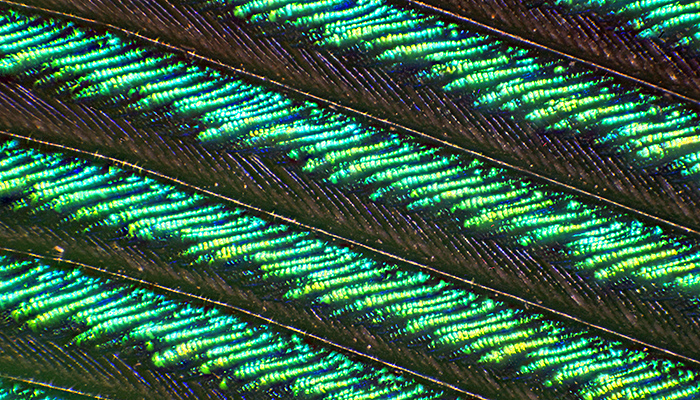
Stacked image of peacock wing feather (Olympus Neo 5× objective, FK 2.5× photo eyepiece, stack of 15 images, 0.6 mm depth of field)
The Obh10B LED illuminator does not only fit the BH-LHM lamp housing for the BH-RLA; it also fits the BH-LH housing for the BHA and BHB stands and fits directly into the back of the BHC stand.
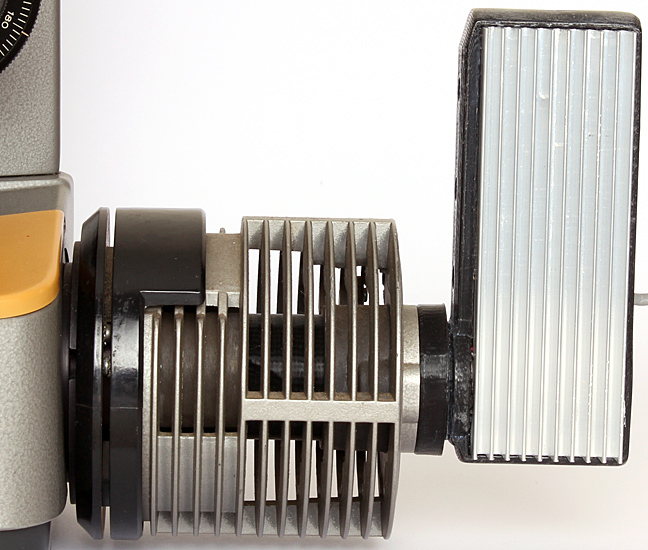
LED conversion of Olympus 30 watt BH-LH lamp house
We tried to get a set of 10 articles for British Science Week, Friday 10th to Sunday 19th March, and just managed it. I wrote 2 of them, mainly using material from the website, on owl pellets and spotted-wing drosophila. I plugged the articles on Facebook and Twitter each day, and we got some nice comments.

Male spotted-wing drosophila
Olympus Zuiko Auto-Macro Lens 38mm f/2.8 @ f/4, 61 images at 40 µm intervals combined in Zerene Stacker
I went to the Reading Convention on Saturday 11th March, always a good event with interesting exhibits and lots of bargains to browse. I restricted myself to a spare bulb for my BH2-MLSH lamp house for £1, and a few Biosil slides at 50p each; I haven’t had time to look at them yet. Mike Samworth kindly gave me a copy of another QMC Monograph to scan for the website, Illuminants and Illumination for Microscopical Work by F. E. J. Ockenden.
I tried to get to Penkridge on Saturday 18th March with Robert Ratford, but we only got to Junction 12 on the M1 before his alternator failed; we had to return on an AA transporter.
February 2017
On 11th February, Chris Thomas and Dennis Fullwood ran a workshop on making dry mounts of hair, fur and feathers attended by some very enthusiastic members. As usual, I was busy taking photographs and making notes and didn’t get a chance to make any slides, but I have since tried photographing hair under brightfield illumination and crossed polarisers, as recommended by Chris.

Human brown hair (brightfield)
Olympus SPlan 40× objective, NFK 2.5× photo eyepiece, 34 images at 1 µm intervals combined in Zerene Stacker
For the photo with crossed polarisers, I rotated the polariser for maximum extinction, and then rotated the stage to obtain the most attractive colours. The normal BH2-SVR stage on my Olympus BH-2 rotates 270°, which is very useful for composing photographs as well as for rotating specimens under crossed polarisers, even though it is not as precise as a proper polarising stage.
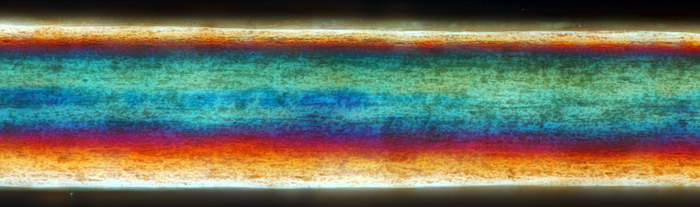
Human brown hair (crossed polarisers)
Olympus SPlan 40× objective, NFK 2.5× photo eyepiece, 28 images at 1 µm intervals combined in Zerene Stacker
I used to use a photographic polarising filter as the polariser (on top of the light outlet on the base), and a cheap disc of polarising film from eBay as the analyser (in the recess where the head’s dovetail fits). I eventually found the proper Olympus A-POL polariser and B-AN analyser at reasonable prices, and they give better extinction and no colour bias.

Olympus A-POL polariser (left) and B-AN analyser
January 2017
Back in 1980 when Olympus replaced the BH compound microscope with the BH-2, they kept the mounts for the heads and nosepieces but changed the mounts for the stages and condensers. While trying out the vertical illuminator from my newly-acquired BHMJ on my BH-2, I discovered that Olympus also kept the 30 mm fitting for lamp houses on their metallurgical microscopes.
So either of these lamp houses:
- BH-LHM lamp house (6-volt 15-watt)
- BH2-MLSH lamp house (12-volt 50-watt)
can be used with any of these illuminators
- BH-MA Brightfield Reflected Light Illuminator
- BH-RLA Brightfield/Darkfield Reflected Light Illuminator
- BH2-MA Brightfield Vertical Illuminator
- BH2-RLA Brightfield/Darkfield Vertical Illuminator.
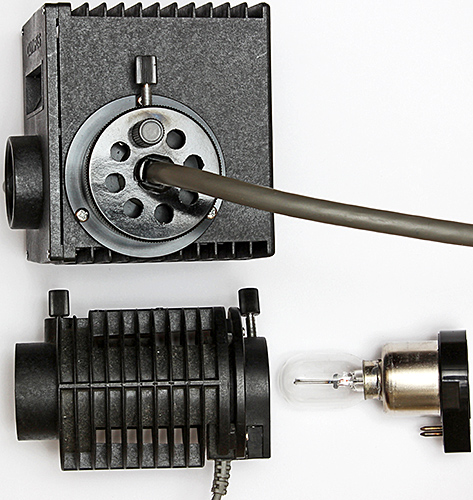
Olympus BH2-MLSH (top) and BH-LHM lamp houses

Olympus BH2-MA Brightfield Vertical Illuminator (top) and BH-RLA Brightfield/Darkfield Reflected Light Illuminator
The collector lens is longer on the BH illuminators, and so the BH2-MLSH lamp house does not slide all the way on.
The lamp houses need different transformers, the TE-II, TF or TGHM for the BH versions and the TGH for the BH-2 versions. The metallurgical objectives must be used with the matching illuminators; BH objectives with BH-MA or BH-RLA illuminators and BH-2 objectives with BH2-MA or BH2-RLA illuminators.
Commenting on this blog
If you would like to comment on anything in this blog, please send me a message.